Fallopian Tube Cancer
Authors
INTRODUCTION
It is well appreciated that conditions involving the fallopian tubes are frequent events prompting medical attention. For example, more than 2 million cases of acute and chronic salpingitis and 75,000 cases of tubal ectopic pregnancy are diagnosed annually in the United States.1 In contrast to this relatively high frequency of nonneoplastic pathologic conditions, primary neoplastic events occur rarely. In fact, primary carcinoma of the fallopian tube is the rarest malignancy of the genital tract.2,3 Therefore, it is not surprising that the majority of information disseminated about benign and malignant primary neoplastic conditions of the oviducts comes from reviews of case reports and small registry data series. Although this lesion is rare, its histologic similarity to ovarian epithelial cancer and its embryologic relationship to the uterus suggest that careful review may provide clues to other more common gynecologic malignancies.
EMBRYOLOGY
The fallopian tube arises embryologically from a common pathway organized to form the uterus, cervix, and upper third of the vagina.4 The process, initiated during the second month of development, starts by anteromedial rotation of the mesenchyme of the urogenital ridge. The paramesonephric ducts, which arise from the anterolateral margin of this invagination, meet in the midline, dividing the pelvic cavity into an uterovesical and uterorectal pouch. Three parts of the paramesonephric ducts are recognized during this process: a proximal (cranial) vertical portion, which opens directly into the coelomic cavity; a middle (horizontal) part, which crosses the mesonephric duct; and a distal (caudal) vertical part, which fuses with its partner from the opposite side. The first two portions develop into the uterine tube, and the latter develops into the uterine/upper vaginal canal. Tissue below the middle horizontal portion, lateral to the fused distal section, develops into the broad ligament. Histologically, remnants of atretic wolffian tissue can be identified there.
Mesenchymal tissue gradually forms a muscular coat around the duct and peripheral coelomic tissue covers the serosal surface. Thus, tubal epithelium is closely related to endometrial and endocervical epithelium. The contiguous smooth muscle that surrounds it is also of common descent. The surface epithelium of the tube is similar to that seen overlying the ovary. It is somewhat paradoxical that carcinomas arising from this organ's epithelium are often described as being of closer histologic likeness to the ovary than to the uterus.
ANATOMIC CONSIDERATIONS
Grossly, the fallopian tube is a hollow, muscular organ capable of stretching several cm beyond its average length of 12 cm. It is classically divided along its length into four sections: pars interstitialis, isthmus, ampulla, and infundibulum. The proximal section is not visible externally, and the latter section is often in direct juxtaposition with the ovary. The tubal lumen varies in width along its length, measuring approximately 1 mm in the pars interstitialis to 4 mm in the ampulla/infundibulum. Histologically, the outer serosal surface is covered with a single layer of mesothelial cells continuous with the visceral peritoneum. Beneath the serosal layer lies the larger tubal muscularis. This layer is composed of an outer longitudinal layer and an inner circular layer that are responsible for much of the muscle mass of the tube. In the region of the pars interstitialis, an inner longitudinal layer is present. The innermost section is composed of deep longitudinal folds, called plicae, which are lined with tall, simple, columnar epithelium. This mucosal epithelium is composed of ciliated, secretory, and intercalary cells and overlies a scanty lamina propria containing vessels and angular cells. The ciliated and nonciliated cells are responsible for motility and secretory activities and can undergo both squamous and mucinous metaplastic changes isolated from, but not dissimilar to, changes in the uterine cervix.5 Mucinous metaplasia has been associated with the Peutz-Jeghers syndrome and may represent a marker for multifocal mucinous neoplasia.6,7 Both ciliated and secretory cells, in response to estrogen stimulation, are at a peak during the menstrual mid cycle and rapidly decrease during the secretory phase or in the environment of high progestin levels, such as during pregnancy or as a result of progesterone therapy.8 The recognition of this relationship has advanced the search for steroid receptors in fallopian tube carcinoma and to explore their link to prognosis or as a modality for therapy.
EPIDEMIOLOGY/ETIOLOGY
Worldwide, more than 1500 cases of primary fallopian tube carcinoma have been reported. The prevalence is estimated to be between .15% and 1.8% of all primary female genital neoplasms.2,3 Data from population-based tumor registries have suggested that the incidence of approximately 3 in 1,000,000 women is consistent among several regions of the United States and abroad and has been stable over the past 50 years.3,9,10 Racial variations have been suggested, with the disease occurring slightly more frequently among whites.9 Age-specific incidence follows a pattern similar to ovarian and endometrial cancers, with a rapid increase in relative frequency after menopause. The median age at diagnosis for fallopian tube carcinoma is 56.7 years (range: 51 to 63 years).2,3,11,12,13 The disease is distinctly uncommon in women younger than 20; more than 65% are menopausal at diagnosis.12,13 There has been an association with nulliparity among reported cases, occurring at a frequency of 27% to 35%; salpingitis, a confounding co-factor, has also been reported.10,14,15,16,17
A recent review of the National Cancer Institute's Surveillance, Epidemiology, and End Results database revealed 416 women with fallopian tube carcinoma diagnosed between 1990 and 1997. For comparison, 9032 women had epithelial ovarian cancer diagnosed between 1991 and 1997. This review also showed an improved survival, when adjusting for stage, for fallopian tube carcinoma compared with epithelial ovarian cancer.18
Histopathologically, tubal epithelium in response to Mycobacterium tuberculosis infection mimics the features of tubal carcinoma and has been implicated as a potential etiologic factor.19 However, in two recent case-control studies, the occurrence of M. tuberculosis infection was no more frequent among patients with fallopian tube carcinoma than in the general population.20,21 It is unclear from the available information whether these factors are independently important in terms of the occurrence of tubal neoplasia. Currently, the etiology of fallopian tube carcinoma is unknown.
Recently, a genetic component of fallopian tube carcinoma has been identified. Small increase has been seen in the incidence of ovarian cancer and early-onset breast cancer in first-degree relatives of women with fallopian tube carcinoma. Several of the fallopian tube carcinoma patients were positive for either the BRCA1 or BRCA2 mutations.22 Also, the finding of occult fallopian tube carcinoma at the time of prophylactic oophorectomy has led to the suggestion that perhaps hysterectomy should be part of this procedure.23
PATHOLOGY
Gross
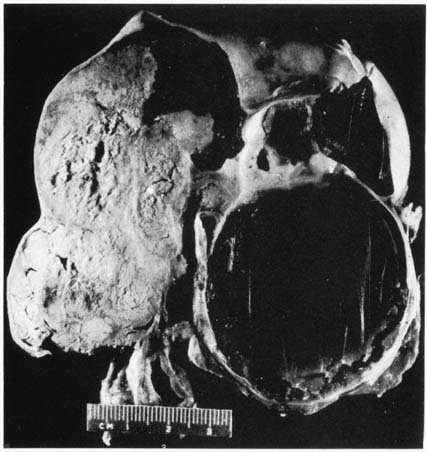
Primary fallopian tube neoplasms are principally characterized by gross tubal distortion (Fig. 1). When the neoplasm is confined to the mucosal or luminal areas, the tube appears fusiform, having a smooth serosal surface. On surgical examination, it may appear to be a hematosalpinx or hydrosalpinx. Alternately, it may convolute and appear to be a tubo-ovarian complex not dissimilar to a tubo-ovarian abscess. On sectioning, a papillary or multinodular mass will be identified along with thick turbid fluid. When the tumor invades through the serosa, grossly exophytic tumor with associated adhesions is common. In these situations, differentiation from primary malignancies of adjacent structures, such as the ovary and uterus, become difficult or impossible.
Characteristically, the tumor is unilateral, located in the infundibulum, and associated with a closed fimbriated end. There is no reported difference in side of involvement; however, in approximately 5% to 30% of cases, bilateral involvement is identified.14,17,24,25,26,27,28,29 This has been explained either as a manifestation of a multifocal process occurring throughout the upper genital tract or as a metachronous primary arising in response to a common etiologic event.11,30 In a Roswell Park series, investigators distinguished patients with carefully defined fallopian tube carcinoma from those who did not meet this criteria. Patients with bilateral involvement had evidence of tubal carcinoma in situ or a transition from carcinoma in situ to invasive carcinoma. This observation suggested that multifocal independent neoplastic processes were likely to have contributed to the field effect.30 In contrast, based on an observation that the rate of bilaterality increases with increasing stage, Schiller and Silverberg31 suggested that a metastatic pathway must also be of importance in explaining bilaterality. This group demonstrated bilateral involvement in only 7% of stage 0 to stage II lesions compared with 30% in stage III and stage IV lesions.
Microscopic
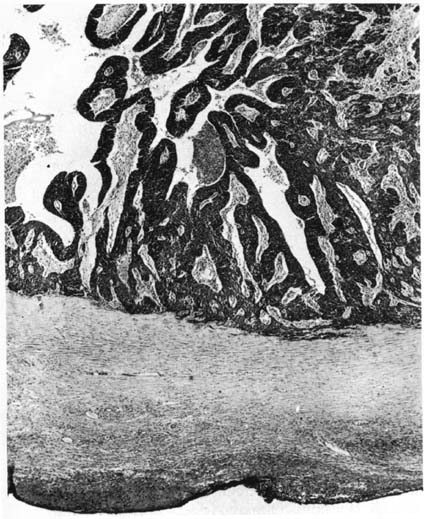
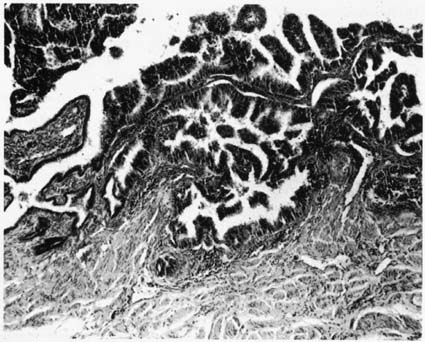
Histologically, carcinoma of the fallopian tube is similar to carcinoma of the ovary. More than 85% of all cases of fallopian tube carcinoma are papillary serous adenocarcinomas, although virtually all epithelial neoplasms of the ovary have been reported as arising in the fallopian tube. A list of reported tumors is provided in Table 1. Morphologically, the papillary structures are preserved and prominent. There is crowding and a disorganized piling up of cells, with frequent mitotic figures. Nuclear atypia is marked, and necrosis is commonly identified (Fig. 2). Capillary and lymphatic space invasion is a process identified early in tumor development. Muscularis invasion also occurs frequently and portends a poorer prognosis when identified.31 Tumor grade has historically been reported but inconsistently relates to prognosis. Criteria outlined by Hu and colleagues categorized these tumors into papillary, papillary-alveolar, and alveolar-medullary patterns.32 An example of the alveolar pattern is demonstrated in Figure 3. Although grading tumors on the amount of glandular formation is a more common technique today, no true glands exist in the tube, and gland-like formation results from excessive proliferative growth and adenomatous change. The majority of lesions so graded are moderately or poorly differentiated. Several series have been unable to establish a prognostic value to this grading system, most likely because of the poor reproducibility of these systems.3,10,14,28,31,32
Table 1. Reported Lesions Involving the Fallopian Tubes
| Inflammatory/Metaplastic | Benign Neoplastic | Malignant Neoplastic |
| Acute and chronic salpingitis | Inclusion cysts | Carcinoma in situ |
| Endometriosis | Epithelial papilloma | Adenocarcinoma |
| Granulomatous salpingitis | Leiomyoma | Sarcoma |
| Tuberculosis | Adenomatoid tumor | Leiomyosarcoma |
| Actinomycosis | Hemangioma | Chondrosarcoma |
| Parasitic | Lipoma | Carcinosarcoma |
| Schistosomiasis | Angiomyolipoma | Metastatic tumors |
| Hydatid disease | Neural tumors | Lymphoma |
| Sarcoidosis | Benign teratoma | Trophoblastic disease |
| Crohn's disease | Adnexal tumor of wolffian origin | |
| Foreign body reaction | ||
| Salpingitis isthmica nodosa |
|
|
Immunohistochemical assays have also been performed in an attempt to identify potentially unique markers of tubal epithelial differentiation, but they have lacked specificity in the reported series. A recent study of a monoclonal antibody assay directed against tubal surface OC-125 antigen (CA125 stain) was positive in 13 of 15 (87%) patients with fallopian tube carcinoma.30 In this trial, serum CA125 levels correlated with staining intensity, but the degree of staining was not related to stage, grade, or survival. Another antigen common to the digestive tract, CA19-9, has been studied. This sialyl-Lewis A antigen is a constitutive glycoprotein of normal cells and is overexpressed in many mucinous neoplasms. One group studying the presence of the antigen in müllerian duct derivatives found it detectable in a higher frequency than CA125 but had an inverse relationship to differentiation of tubal carcinomas.33 Thus, little new specific information regarding tubal differentiation is available.
RARE TUMORS OF THE FALLOPIAN TUBE
Epithelial
Several nonserous epithelial tumors have been reported as primary neoplasms in the tube.34,35,36,37 These include endometrioid with or without benign and malignant squamous components, clear-cell, mucinous, squamous cell, and transitional cell carcinomas. With the exception of the latter group, no difference in clinical behavior has been recognized with these variants. Recently, Uehira and coworkers34 studied the behavior of 12 transitional cell carcinomas of the fallopian tube and compared them with nine nontransitional cell carcinomas of the fallopian tube. Although controlled for treatment and stage, transitional cell carcinomas tended to relapse later (31.2 months vs. 14.4 months), resulting in a superior 2-year survival rate. This has been observed in similar histologic tumors of the ovary.38
Stromal/Germ Cell
Rarely, stromal and germ cell ovarian lesions have been reported in extraovarian sites. By far, the majority of these in the oviduct are gestational choriocarcinoma.39,40 The clinical and pathologic feature of this neoplasm is similar to uterine choriocarcinoma. The most common etiology is from an ectopic pregnancy. Sex cord tumor with annular tubules was reported in a fallopian tube associated with a focus of endometriosis.41 Immature teratoma and struma salpingis have been described, often associated with a separate ovarian lesion.42
Sarcoma
Sarcoma of the fallopian tube is not dissimilar to that arising in the uterus. The most common of these is the carcinosarcoma.43,44,45 Approximately 55 cases have been reported. Commonly, they are subclassified into heterologous and homologous components with equal representation. Leiomyosarcoma, angiosarcoma, and fibrosarcoma are rarely defined entities, representing less than 3% of all primary fallopian tube neoplasms.46,47,48
Other Tumors
Female adnexal tumors of probable wolffian origin are rare tumors arising from the distal tube or broad ligament and present as encapsulated solid or complex masses that may attain a large size. Microscopically, the lesions have closely packed tubules, sieve-like spaces, and diffuse proliferation. The cells lack mitotic activity and behave in a benign pattern. Approximately 20 of these tumors have been reported.49
Secondary Tumors
The most common neoplastic process involving the fallopian tube are secondary tumors. One estimate from the Johns Hopkins Hospital reviewing 180 tubal malignancies found that 80% of these were secondary.50 Most frequently, the origin is from the ipsilateral or occasionally contralateral ovary. Up to 50% of ovarian carcinomas will involve the tube, and approximately one fifth of serous neoplasms apparently confined to the ovary will have occult metastatic involvement in serially sectioned tubes.51 Direct tumor invasion is the most common venue. Similarly, albeit at a lower frequency, direct or metastatic involvement of the tube from tumors of the uterine corpus and cervix is seen in advanced lesions of these primary sites. As previously mentioned, direct extension along the mucosal surface may be continuous from the corpus and/or cervix. The most frequent histologic patterns in this situation are squamous cell carcinoma or mucinous carcinoma. Rarely, squamous carcinoma in situ can extend from the cervix into the tube.52 Other reported primary tumors that metastasize to the tube are breast carcinoma, colon carcinoma, multiple myeloma, osteosarcoma, and bladder carcinoma.17,53,54
Classification
In light of the obvious similarities at presentation and often local involvement of the ipsilateral adnexa by fallopian tube carcinoma, investigators have sought to develop precise histologic criteria to aid in the distinction of the primary's origin. The first generally accepted pathologic criteria were defined by Hu and associates32 in 1950: on gross inspection, the main tumor is in the fallopian tube; on microscopic inspection, the mucosa is involved and shows a papillary pattern; and if the tubal wall is involved extensively, transition between benign and malignant epithelium should be demonstrable.
The following criteria, modified by Sedlis in 1978,55 are now generally accepted as the principle diagnostic criteria for fallopian tube carcinoma: the primary tumor arises from the endosalpinx; the histologic pattern reproduces the epithelium of the tubal mucosa; transition from benign to malignant epithelium is seen; and the ovaries and endometrium are either normal or contain less tumor than in the tube.
It is likely that the true incidence of primary fallopian tube carcinoma is underestimated by these rigid criteria, but identified cases will have a consistent diagnostic pathway allowing more precise informational analyses.
CLINICAL CHARACTERISTICS
Natural History
Insight into the process by which fallopian tube carcinoma progresses is ascertained by careful inspection of tumor spread at initial operation. Despite its commonalty with the uterine corpus, advanced primary fallopian tube carcinoma more closely mimics ovarian carcinoma with respect to spread patterns. Commonly, extratubal disease is identified throughout the peritoneal cavity by the presence of multiple peritoneal deposits and associated ascites. The mechanism by which this occurs is somewhat debatable, but it has been attributed to tumor exfoliation. Because tubal ostia are commonly closed at diagnosis, direct extension through the muscular wall must contribute as a predominate source. Evidence to support the importance of muscular invasion has been suggested and has been linked to survival.17,31 This has championed a staging scheme similar in part to the Dukes classification of colon carcinoma by one group.31
Recently, greater attention has been given to the role of lymphogenous spread patterns. In their classic manuscript of genital lymphatics, Plentl and Friedman56 described two pathways of lymphogenous drainage for the fallopian tube. The primary route drains into the subovarian plexus, through the infundibulopelvic ligament, and terminates in the region of the renal veins. A second pathway is established through the lower broad ligament and round ligament lymphatic streams. Tumor emboli through these pathways will create deposits along the internal iliac and presacral chains, the external iliac, and occasionally the inguinal lymphatics. The propensity for early lymphatic involvement has only recently been appreciated, as formal surgical staging now is performed in most new cases of primary fallopian tube carcinoma. Table 2 and Table 3 outline the frequency of reported lymphatic metastases among patients undergoing surgical staging at diagnosis and those explored at the time of recurrence, respectively.31,57,58,59,60,61,62,63 Overall, more than 35% of all new cases will have evidence of lymphatic spread at presentation. Several authors have stressed the importance of routine sampling. These reports have documented patients in whom isolated para-aortic metastatic disease was the only site of extratubal disease at presentation and patients undergoing secondary exploratory surgery in whom para-aortic metastasis was the only site of recurrence.31,57,62
Table 2. Frequency of Lymphatic Metastases in Surgically Staged Primary Fallopian Tube Carcinoma at Initial Operation
| Author | No. Patients | Nodal Metastases (%) |
| Tamini and Figge57 | 15 | 05 (33) |
| Schray et al58 | 34 | 12 (34) |
| Maxson et al59 | 05 | 02 (40) |
| Klein et al60 | 21 | 09 (43) |
| Barakat et al61 | 06 | 01 (16) |
| Total | 81 | 29 (36) |
Table 3. Frequency of Lymphatic Metastases in Surgically Staged Primary Fallopian Tube Carcinoma at Recurrence
| Author | Nodal Metastases No. Patients | (%) | Comment |
| Rose et al30 | 15 | 10 (67) | Autopsy series |
| Tamini and Figge57 | 15 | 2 (13) | Only site of recurrence |
| Ausmussen et al62 | 15 | 2 (13) | Only site of recurrence |
| Maxson et al59 | 08 | 5 (63) | Recurrence sites |
| Semrad et al63 | 14 | 7 (50) | Second-look data |
Presentation
In most reported series, patients with fallopian tube carcinoma are symptomatic at presentation. Eddy and colleagues15 and Peters and associates17 reported that only 6% and 9% of patients, respectively, were asymptomatic on presentation. Classically, two triads of symptoms have been described, and at least one syndrome is considered pathognomonic. Unfortunately, as in other pathologic conditions with classic symptomatology, only a fraction of the cohort will present in this manner. Latzko's triad, described in a 1916 publication, is the combination of pelvic pain, a pelvic mass, and serosanguineous vaginal discharge.64 A second triad, with a reported frequency of 6% to 38%, involves vaginal bleeding, vaginal discharge, and lower abdominal pain.16,65,66 In addition, the syndrome characterized by colicky lower abdominal pain and a resolving mass after profuse vaginal watery discharge or hydrops tubae profluens, is uncommonly reported (approximately 9%) in large review series, although thought to be diagnostic of fallopian tube carcinoma.11,15,17,31 In many cases, the condition is not reported at all. Individually, the symptoms are nonspecific, general, and nondiagnostic of carcinoma. In one case identified after hysterectomy, the symptoms were misinterpreted as being caused by a vesicovaginal fistula.67
The most common symptom reported in patients with fallopian tube carcinoma is menopausal bleeding. Various authors have reported this symptom in 30% to 68% of newly diagnosed cases.10,15,20,24,25,65,68,69 The next most common symptom is abdominal pain, reported in 31% to 56% of patients. The pain is often colicky, dull, and located in the lower abdomen or posterior pelvis. Pain is thought to result from tubal distention and from peristaltic activity of the organ in an attempt to pass the turbid fluid. Vaginal discharge, reported in approximately 20% of patients, may result from the successful evacuation of the tubal contents. Other symptoms, such as intraperitoneal bleeding, urinary urgency, abdominal distention, and pelvic pressure, are less commonly reported and nonspecific. Unusual presentations in unsuspected cases have followed from symptomatic metastatic foci. Reports of respiratory insufficiency, paraplegia, ataxia, blindness, facial numbness, and umbilical drainage have prompted evaluation identifying a common primary lesion in the fallopian tube.66,70,71,72,73 Despite the nonspecific symptomatology, most patients with fallopian tube carcinoma present with their symptoms far sooner than patients presenting with ovarian carcinoma. In the series by Eddy and associates,15,74 50% of diagnosed cases presented with symptoms of 2 months or less, and nearly three-quarters presented within 6 months of the onset of their symptoms. This early presentation may explain the relatively increased frequency of early stage neoplasms at diagnosis compared with ovarian carcinoma.
On physical examination, the most common finding is a pelvic or abdominal mass, occurring in 61% to 70% of reported series.15,17,69 Ascites is less commonly identified but is a hallmark of advanced disease. Between 15% to 19% of patients will have a normal preoperative examination.15,69 Review of the clinical characteristics in patients with fallopian tube carcinoma demonstrates that most patients will have an indication for exploratory surgical evaluation; however, a correct preoperative diagnosis is infrequently made.
Preoperative Evaluation
Since the 1940s, diagnostic testing has been used to evaluate patients presenting with the symptomatology mentioned earlier. One of the earliest diagnostic procedures championed was hysterosalpingography.11 Although this procedure has the ability to demonstrate abnormalities in tubal anatomy, it lacks sensitivity, especially among patients with chronic inflammatory processes. The theoretic risk of extratubal spread from the procedure also detracted from widespread use. More commonly today, patients presenting with the aforementioned symptomatology undergo evaluation of the pelvis with specific radiographic tools. Ultrasonography has been reported in the diagnostic evaluation of fallopian tube carcinoma since 1978.74 Although transabdominal scans have correctly identified primary neoplasms in the fallopian tube, limited resolution has made it difficult to differentiate among tubo-ovarian complexes, ectopic pregnancy, broad ligament neoplasms, uterine neoplasms, and ovarian lesions.74,75 With the advent of widespread transvaginal ultrasound use, correct identification of fallopian tube pathology and carcinoma is increasing.76,77 This technique allows precise evaluation of tubal characteristics because of the proximity of the transducer to the adnexa. Reported diagnostic features include sausage-shape mass, cystic spaces with mural nodules, a multilocular mass with a cog and wheel appearance, and low-impedance vascular flow within the solid components.78 Both computed tomography and magnetic resonance imaging techniques have been studied in the setting of adnexal pathology evaluation. Although these studies are valuable in visualizing pelvic and abdominal tissue relationships and abnormalities, limited resolution (>1 cm) has hampered precise localization of primary foci, especially in the region of the broad ligament.
All of these techniques rely on differential tissue density to exploit abnormalities in the affected sites. Their limitation lies in visualizing isodense benign and malignant processes. One new modality that explores the differential of biochemical activity is positron emission tomography (PET). The technique involves administering a positron emitting radioisotope; on interacting with electrons, the radioisotope produces 511-keV photons circumferentially, which are detectable. Use of 2-fluoro2-deoxyglucose has become popular because the glucose analog is taken up by normal cellular glucose transport mechanisms and trapped in the cells. High use is seen in malignant processes, which can be identified by hot spots on computer-generated images. Karlan and colleagues79 recently reported the use of PET in a patient with recurrent fallopian tube carcinoma whose sole presenting pathology was an umbilical nodule. After total-body PET evaluation, several other extra-abdominal metastatic sites were identified.
Results of cytologic evaluation of the vagina and cervix and endometrial aspiration have been reported to be abnormal in 30% to 80% of cases of tubal carcinomas, although most reports demonstrate that the abnormalities are nondiagnostic for the neoplasm.17,68,80,81 Cytologic criteria have been described to help differentiate the cytologic characteristic of adenocarcinoma from endometrial lesions, but are commonly accurate in only 20% to 30% of cases.15,17,68,80 Likewise, vaginal pool collection of the profuse watery discharge infrequently contains cellular material for accurate diagnosis. Endometrial aspiration demonstrated adenocarcinoma in 6 of 12 patients reported in the series by Hirai and colleagues,68 although the majority of patients had a preoperative diagnosis of endometrial or ovarian cancer.
Staging
Because of inconsistent acceptance of pertinent prognostic variables and a limited understanding of the disease's natural history, several classification and staging schemes have been advocated. Because the tube is a hollow viscus, Erez and colleagues82 suggested a staging scheme based on the relationship of the tumor to the histologic layers of the tubal wall. The importance of muscularis invasion and extratubal extension brought forth a staging scheme modeled after the Dukes classification of colorectal carcinoma.31,82 Subsequently, Dodson and associates83 suggested, and the International Federation of Gynecology and Obstetrics (FIGO) adopted, a modification of the staging schema for ovarian carcinoma. In 1991, this was further altered to reflect the prognostic significance of muscularis invasion among stage I cancers (Table 4).84 The advantage of this classification is that it is familiar to most treating surgeons, incorporates features of the Erez and colleagues staging system82 and quantifies extratubal disease, which is an important predictor of survival and response to therapy, as will be demonstrated later. The disadvantage is that subcategorization of this exceedingly uncommon tumor makes meaningful interpretation difficult because of low numbers by category.
Table 4. FIGO Fallopian Tube Staging Criteria
| Stage 0: | Carcinoma in situ is limited to tubal mucosa |
| Stage I: | Growth is limited to the fallopian tube |
| IA: | Growth is limited to one tube with extension into the submucosa and/or muscularis, but not penetrating the serosal surface |
| IB: | Growth is limited to both tubes with extension into the submucosa and/or muscularis, but not penetrating the serosal surface; no ascites |
| IC: | Tumor is either stage IA or IB with tumor extension through or onto the tubal serosa; or with ascites present containing malignant cells or with positive peritoneal washings |
| Stage II: | Growth involves one or both fallopian tubes with pelvic extension |
| IIA: | Extension and/or metastasis is to the uterus and/or ovaries |
| IIB: | Extension is to other pelvic tissues |
| IIC: | Tumor is either stage IIA or IIB with ascites present containing malignant cells or with positive peritoneal washings |
| Stage III: | Tumor involves one or both fallopian tubes with peritoneal implants outside of the pelvis and/or positive retroperitoneal or inguinal nodes. Superficial liver metastases equals stage III. |
| IIIA: | Tumor is grossly limited to the true pelvis with negative nodes, but with histologically confirmed microscopic seeding of abdominal peritoneal surfaces |
| IIIB: | Tumor involves one or both tubes with histologically confirmed implants of abdominal peritoneal surfaces, none exceeding 2 cm in diameter. Lymph nodes are negative |
| IIIC: | Abdominal implants are greater than 2 cm in diameter and/or positive retroperitoneal or inguinal nodes |
| Stage IV: | Growth involves one or both fallopian tubes with distant metastases. If pleural effusion is present, there must be positive cytology to be stage IV. Parenchymal liver metastases equals stage IV. |
Patients often present with fallopian tube carcinoma at an earlier FIGO stage compared with patients with ovarian carcinoma; however, up to 50% of the former patients will have extratubal disease on evaluation. This observation is likely due to the higher frequency of symptomatic patients at presentation. Distention of the tube by a luminal mass and fluid is implicated as a cause of this. Unfortunately, the impact of earlier stage disease on survival is not as significant for patients with fallopian tube carcinoma as it is for patients with ovarian cancer.
PROGNOSTIC FACTORS IN FALLOPIAN TUBE CARCINOMA
Delineation of important factors defining the natural history of this neoplasm have chiefly originated from similar studies of patients with ovarian carcinoma. Several factors have been investigated, but limitations caused by small sample size, inconsistent staging, and various treatment protocols have left this aspect of the disease's study incomplete. Clinical and surgical stage, age, race, histology, tumor grade, depth of invasion, lymph–vascular invasion, DNA ploidy, mitotic activity, nuclear grade, inflammatory reaction, estrogen and progesterone receptors, and treatment modality are among the many factors investigated.16,17,30,31,60,85,86 Overall, the most consistently identified prognostic variable for disease-specific survival is surgical stage. When stage is controlled for, however, most specific characteristics of individual tumors fail to attain independent status. From the mentioned variables, only lymph–vascular invasion, depth of muscularis invasion, and inflammatory reaction have been shown to be independent prognostic variables for this disease.
Tamini and Figge57 and Ausmussen and associates63 reported on the importance of lymphatic invasion for both lymph node metastases and overall survival. Ausmussen demonstrated that patients with these features were more likely to die from the disease when present.63 Likewise, Peters and colleagues17 demonstrated that stage I patients with deep (≥50%) muscularis invasion were four-times more likely to die of disease at 8 years follow-up than patients with less invasive lesions. This effect became evident after 5 years of observation. Rosen and colleagues85 reported on 66 surgically staged patients in whom cellular features were evaluated. They found that if an intense inflammatory reaction was observed in the primary tumor, patients survived longer. This effect may be explained by implication of an intense host response. The authors suggested that in this situation, cellular release of cytokines such as tumor necrosis factor-α or transforming growth factor-β may be directed against tumor growth.
MANAGEMENT OF FALLOPIAN TUBE CARCINOMA
As is often the case in uncommonly diagnosed tumors, accurate information regarding proper management is rare. Randomized trials addressing the roles of adjuvant treatment, radical surgery, reassessment laparotomy, and cytoreduction, as well as the most active drugs or proper radiotherapy are, at best, lacking. Most of the information addressing these important questions is either from retrospective or individualized trials or are extrapolated from information gained from the study of ovarian carcinoma. The difficulty of studying fallopian tube carcinoma was expressed by Morris and associates,87 who reported on a phase II adjuvant chemotherapy protocol at the MD Anderson Cancer Center. This study required 9 years to enroll just 18 patients in a consistent treatment plan. They argued that the issue of similarity between fallopian tube carcinoma and ovarian carcinoma should be addressed first so that, if the natural history of fallopian tube carcinoma was consonant with that of ovarian carcinoma, the standard treatment of the latter would be appropriate. The available data regarding surgical goals, adjuvant treatment with radiation and chemotherapy, and reassessment operations are presented next.
Surgical Goals
As previously discussed, the natural history and patterns of spread of fallopian tube carcinoma most closely mimic characteristics of ovarian carcinoma. Therefore, it is not surprising that the surgical goals recommended have been patterned after those now purported for patients with ovarian carcinoma. The surgical goals recommended have been premised on the sensitivity of ovarian carcinoma to adjuvant therapy. Now that a formalized and generally accepted staging format has been published, the surgical goals are clearly to provide enough information to ascertain a surgical stage.84 In light of the tumor's usual presentation, this most often involves removal of the uterus, tubes, and ovaries, along with assessment of the tumor distribution in the peritoneal surfaces and retroperitoneum.
On the basis of the previously mentioned propensity for lymphatic metastases, special attention should be given to proper sampling of the pelvic and para-aortic lymphatic chains. There is currently no evidence to suggest that radical hysterectomy or complete lymphatic dissection adds to progression-free or overall survival unless it contributes to the primary tumor's extirpation. It is not uncommon for patients with otherwise stage I or stage II disease to present with isolated para-aortic metastatic disease. This clinical feature may suggest that lymphatic involvement may precede intra-abdominal metastases. In the series by Tamini and colleagues,57 two of eight patients with lymphatic metastases had disease solely in the para-aortic chain. Upstaging of clinically confined or localized tumors is common. In the review by Schray and colleagues,58 five of nine patients with clinical stage I disease were upstaged on the basis of extrapelvic lymphatic involvement; in the series by Klein and colleagues,59 three of seven stage II cases were upstaged on the basis of lymphatic metastases. Review of these data underscores not only the importance of systematic evaluation, but also the impact of accurate disease status on treatment decisions. One investigator suggested that patients who have not been properly staged or explored may undergo successful lymphatic sampling by laparoscopy.88
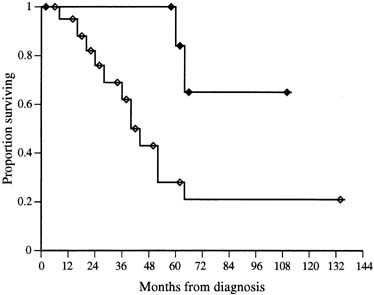
The role of cytoreductive surgery has been the focus of several reviews and appears to confer the same progression-free survival enhancement as is seen among patients with ovarian carcinoma.15,16,17,61,89 Many reports have used differing definitions of optimal cytoreduction, but the most profound and consistent difference is seen among patients with no residual disease after attempted cytoreduction. Barakat and colleagues61 noted among 31 stage II to stage IV patients treated with platinum-based adjuvant therapy, those with no residual disease after a maximal cytoreductive effort had a 65% 5-year survival, compared with a 19% 5-year survival in those described as having any residual(Fig. 4). Similarly, in a review of 71 patients treated at the MD Anderson Cancer Center with platinum-based and nonplatinum-based chemotherapy and/or radiation therapy, those with no residual disease had a 5-year survival of 29% compared with 15% for those with residual disease less than 2 cm and 7% for residual disease equal to or greater than 2 cm.15 In a review of 18 patients treated at the same institution with platinum-based chemotherapy, Morris and associates87 noted that among 15 patients with stage II to stage IV disease, five of six patients (83%) with no residual were without disease at follow-up (median survival not reached at 96 months) compared with only three of nine patients (33%) with any residual at a median 29 months. Considering the small sample size, disparate adjuvant therapies, and lack of information on biologic aggressiveness, it is still appreciated that the effect of complete tumor resection will likely connote a longer progression-free survival among patients with stage II to stage IV disease.
Second-look laparotomy has also been evaluated in this patient population (Table 5).15,16,30,57,68,87,90,91,92,93,94,96,97 Guidelines as to use of the procedure and impact on survival are less well understood and studied than among patients with ovarian cancer. Further, because of unspecified selection criteria, variant types of adjuvant therapy, and lack of complete surgical staging data, interpretation among patients undergoing the operation cannot be precise. Certain generalizations may be inferred, however, on review of the more than 90 reported operations performed since 1980. First, findings at this operation seem to provide some meaningful information about recurrence and progression-free survival. Among the 58 negative second-look operations reported, only 12 (20.6%) have recurred. Second, sixty-three percent of the operations were pathologically negative, although this was most frequently among stage I, grade 1 cases. This operation is probably not important in this subgroup. The most consistent prognostic indicator of outcome in this operation is the amount of residual disease at primary cytoreduction. Third, secondary cytoreduction on the identification of gross residual disease31,61 probably has little or no effect on overall survival, because no survival advantage was seen among patients with gross residual disease after second cytoreduction and those with microscopic residual.
Table 5. Outcomes in Second-Look Laparotomy Procedures of Primary Fallopian Tube Carcinoma
| Author | No. of Second-Look No. Patients | No. (%) of Negative Procedures | No. (%) of Recurrences After Procedures | Negative Procedure |
| Pectasides et al97 | 017 | 09 | 6 (67)0 | 1 (17) |
| Barakat et al96 | 035 | 35 | 21 (60)00 | 4 (19) |
| Morris et al87 | 018 | 08 | 4 (50)0 | 0 (0)0 |
| Rose et al30 | 064 | 08 | 2 (25)0 | 0 (0)0 |
| Podratz et al16 | 047 | 01 | 0 (0)00 | 0 (0)0 |
| Brown et al95 | 021 | 04 | 4 (100) | 2 (50) |
| Peters et al17 | 046 | 07 | 6 (86)0 | 2 (33) |
| Deppe et al93 | 004 | 02 | 2 (100) | 0 (0)0 |
| Jacobs et al92 | 009 | 03 | 3 (100) | 0 (0)0 |
| Roberts et al91 | 028 | 02 | 2 (100) | 1 (50) |
| Harrison et al90 | 036 | 05 | 1 (20)0 | 0 (0)0 |
| Hirai et al68 | 015 | 01 | 1 (100) | 0 (0)0 |
| Eddy et al15 | 071 | 08 | 5 (63)0 | 2 (40) |
| Tamini and Figge57 | 015 | 01 | 1 (100) | 0 (0)0 |
| Total | 426 | 92 | 58 (63)00 | 12 (21)0 |
In summary, evaluation of disease response to primary therapy may be indicated on prognostic grounds alone in patients with stage II to stage IV disease; however, little additional benefit is gained by secondary cytoreduction among patients without a complete response to primary therapy.
Radiation Therapy
Supported by early studies suggesting the sensitivity of fallopian tube cancers to radiation, this modality had been considered a principle asset of adjuvant therapy.83,98 Several delivery mechanisms have been evaluated, including intraperitoneal radiocolloids, external orthovoltage and megavoltage radiotherapy, brachytherapy, and radiotherapy with chemotherapy. Much of the information regarding individual therapies comes in the context of inconsistent staging information, palliative versus curative goals, and variable treatment fields (whole abdomen vs. pelvic). In addition, although this disease is sensitive to the effects of radiation, this modality may not prevent tumor recurrence. Generalizations regarding the role of radiotherapy can be made, but specific recommendations as to the modern treatment of fallopian tube carcinoma are difficult given the lack of information.
As the natural history of fallopian tube carcinoma becomes clearer, it is reasonable to assume that radiotherapy will fail in all stages, except in cases in which the disease is completely resected or limited. Among this group of patients, intraperitoneal radiocolloids and pelvic radiation therapy have been popular, albeit with mixed response results.
After administering adjuvant intraperitoneal 32P with or without pelvic radiotherapy, Phelps and Chapman98 reported eight long-term survivors out of nine stage I/II patients. Likewise, Schray and colleagues,58 using adjuvant intraperitoneal 32P alone, reported long-term survival in two patients with stage I/II disease. Other trials have not consistently reflected these earlier encouraging results. For example, in a report from the Mayo Clinic, Podratz and colleagues16 found recurrence in four of six patients with stage IA, IB, and IIA disease after pelvic radiation and in five of five patients with stage IC, IIB, and III disease after whole-abdominal radiation. Further, in a review of 115 fallopian tube cancer patients, 39 of whom had stage I disease, no survival benefit could be established with the use of adjuvant radiation over surgery alone.17 This effect held true for stage II patients as well, although there was a clear treatment bias among this high-risk group. Likewise, in the review from the M.D. Anderson Cancer Center, no benefit could be demonstrated in the use of adjuvant radiotherapy over surgery alone in 20 stage I and stage II patients.15 Morbidity from therapy was significant: of 43 patients, seven required hospitalization, three underwent intestinal resection, and one died of intestinal perforation. Despite these observations, the high rate of recurrence in this group of patients calls for some form of adjuvant therapy. These authors agree that patients with intra-abdominal risk or gross intra-abdominal disease should, in general, be treated with systemic chemotherapy. Only selected patients with microscopic extrapelvic disease should be considered for adjuvant therapy with whole-abdominal radiation.
Chemotherapy
Much of the recent advancements in the treatment of fallopian tube carcinoma has come from the evaluation of adjuvant single and multi-agent platinum-based chemotherapy. Although chemotherapy has been implemented in this disease since its advent, little success was achieved in early trials. Many of these regimens consisted of single-agent alkylating or anthracycline-based combinations and were administered for recurrent or rapidly progressing disease, often after adjuvant radiation therapy. Table 6 depicts trials of noncisplatin-based treatment regimens in the adjuvant setting for advanced disease. Although a few partial responses were seen, long-term survivals were rare, ranging from 0% to 29%. The most extensive experience among the single-agent adjuvant trials were the alkylators, such as melphalan, cyclophosphamide, chlorambucil, and thiotepa. Combination regimens reported often added doxorubicin to cyclophosphamide. In one series, the benefit of a multi-agent nonplatinum-based regimen over single-agent therapy was clear, demonstrating a three-fold increase in 5-year survival, from 9% to 29%.94
Table 6. Summary of Series Reporting Responses to Nonplatinum-Based Chemotherapy in Stage II to Stage IV Fallopian Tube Carcinoma
| Author | 5-Year No. Patients | Survival (%) |
| Denham and MacLennan14 | 39 | 18 |
| Eddy et al15 | 32 | 5 |
| Muntz et al67 | 1 | 0 |
| Peters et al94 | 7* 23 | 9*29 |
| Pfeiffer et al10 | 17 | 6 |
| Podratz et al16 | 18 | 19 |
| Roberts et al91 | 31 | 6 |
| Total | 168 | 13.2 |
*Single-agent protocol versus a multiagent noncisplatin-based protocol
With the advent of cisplatin and the promising results seen in early trials of patients with epithelial ovarian cancer, renewed interest developed in treating patients with fallopian tube carcinoma. The first documented complete responses with this agent were credited to Deppe and colleagues,93 who documented a complete response in two of four patients with advanced, measurable disease. Since this report, several retrospective series have been published studying cisplatin-containing regimens in the adjuvant setting, with response rates ranging from 21% to 91% and 5-year survival ranging from 14% to 51% (Table 7). This is comparable with responses seen in similarly staged patients with ovarian carcinoma. There has been only one prospective trial reported, which took 9 years to accumulate 18 patients.87 Information supporting the relationship of specific responses to cisplatin-based chemotherapy with residual tumor volume is lacking, but such a relationship was suggested by Barakat and associates.61 In their review, 5-year survival among 14 stage II to stage IV, cisplatin-treated patients with no measurable disease was superior to that of patients similarly staged and treated, but with measurable residual disease (83% vs. 29%). Most reported regimens incorporating cisplatin commonly add an alkylator, such as cyclophosphamide alone or in combination with an anthracycline. Data supporting the use of adjuvant chemotherapy in low-risk patients (i.e., those with stage IA or stage IB disease) are lacking and insufficient for recommendations. Criteria for treatment in this subgroup will likely follow that of ovarian carcinoma, although it is important to recognize the lack of association of grade and the importance of muscularis penetration in making decisions for adjuvant therapy.
Table 7. Summary of Series Reporting Responses to Platinum-Based Chemotherapy in Stage II to Stage IV Fallopian Tube Carcinoma
| Author | No. Patients | Overall Response (%) | 5-Year Survival (%) |
| Barakat et al61 | 31 | 60 | 51 |
| Gurney et al89 | 10 | 80 | 18 |
| Maxson et al59 | 12 | 92 | 27 |
| Morris et al87 | 18 | 53 | 46 |
| Muntz et al67 | 12 | 71 | 50 |
| Pectasides et al97 | 11 | 91 | 48 |
| Peters et al94 | 16 | 81 | 15 |
| Rose et al30 | 14 | 21 | 14 |
| Total | 124 | 68.5 | 36.1 |
More recently, with the introduction of carboplatin and its gradual replacement of cisplatin in ovarian cancer treatment, interest in carboplatin in fallopian tube carcinoma has arisen. Many ovarian cancer trials, of all phases, studying carboplatin-containing regimens also include fallopian tube carcinoma. They show excellent response rates (81%) but most often there is no subgroup analysis of fallopian tube carcinoma available.99,100 Unfortunately, there is a high incidence of hypersensitivity to carboplatin (5% to 67%), which increases with the number of cycles. As platinum-containing regimens seem to be the most active in this condition there is considerable interest in retreating patients with a platinum agent after carboplatin hypersensitivity. Multiple studies have demonstrated the feasibility and safety of readministeration in this setting.101,102
Future trials will certainly incorporate the new taxanes, such as paclitaxel and docetaxel, and newer topoisomerase I inhibitors, such as topotecan and camptothecin, because these agents have recently demonstrated promising data among patients with advanced ovarian carcinoma.104,105 A review of 23 patients who received primary paclitaxel-containing regimens, the majority also received a platinum agent, revealed overall median progression-free survival of 27 months.103 A phase I trial of paclitaxel, melphalan, and cisplatin in patients with advanced fallopian tube cancer has been reported and is entering phase II study.106
Hormonal Therapy
Use of hormonal therapy in both the adjuvant and salvage setting has been reported in many series, although not independently.14,15,16,23,30,107 The rationale is supported by information relating the cyclic influence of hormones on tubal epithelium. The routine use of these agents cannot be advocated currently, however, because of the lack of association between estrogen and progesterone receptor status and prognosis and the low responses observed clinically with the use of megestrol acetate or medroxyprogesterone acetate.85
SUMMARY
Fallopian tube carcinoma is a rare disease entity of the female genital tract. Evaluation of its natural history is not well understood, but with stricter adherence to the guidelines for staging and categorization of risk factors, insight into the best treatment approach will likely follow. Given this disease's rarity, it is probably most appropriate to exploit this avenue before calling for randomized phase III trials, because even multicenter research bases will require too long a time for adequate evaluation of new agents or protocols.
REFERENCES
Herbst A, Mishell D, Stenchever M et al: Upper genital tract infections. In: Herbst A, Mishell D, Stenchever M, Droegemueller W, (eds): Comprehensive Gynecology. p 691, St. Louis, Mosby-Year Book, 1992 |
|
Makien J, Grenman S, Klemi P: Diagnostic aspects of primary tubal malignancy. Acta Obstet Gynecol Scand 65:507, 1986 |
|
Rosen A, Klein M, Lahousen M et al: Primary carcinoma of the fallopian tube: A retrospective analysis of 115 patients. Br J Cancer 68:605, 1993 |
|
Sadler T: Urogenital systems. Langman's Medical Embryology. p 247, Baltimore, Williams & Wilkins, 1985 |
|
Woodruff JD, Julian CG: Multiple malignancy in the upper genital canal. Am J Obstet Gynecol 103:810, 1969 |
|
Fetissof F, Berger G, Dubois MP et al: Female genital tract and Peutz-Jehgers syndrome: An immunohistochemical study. Int J Gynecol Pathol 4:219, 1985 |
|
Seidman JD: Mucinous lesions of the fallopian tube: A report of seven cases. Am J Surg Pathol 18:1205, 1994 |
|
Flickinger GL, Meuchler EK, Mikhail G: Estradiol receptor in the human fallopian tube. Fertil Steril 25:900, 1974 |
|
Rosenblatt KA, Weiss NS, Schwartz SM: Incidence of malignant fallopian tube tumors. Gynecol Oncol 35:236, 1989 |
|
Pfeiffer P, Mogensen H, Amtrup R et al: Primary carcinoma of the fallopian tube: A retrospective study of patients reported to the Danish Cancer Registry in a five-year period. Acta Oncol 28:7, 1989 |
|
Nordin AJ: Primary carcinoma of the fallopian tube: A 20-year literature review. Obstet Gynecol Surv 49:349, 1994 |
|
Gatto V, Selim MA, Lankerjani M: Primary carcinoma of the fallopian tube in an adolescent. J Surg Oncol 33:212, 1986 |
|
Schink JC, Lurain JR: Rare gynecologic malignancies. Curr Opin Obstet Gynecol 3:78, 1991 |
|
Denham JW, MacLennan KA: The management of primary carcinoma of the fallopian tube. Cancer 53:166, 1984 |
|
Eddy GL, Copeland LJ, Gershenson DM: Fallopian tube carcinoma. Obstet Gynecol 64:546, 1984 |
|
Podratz KC, Podczaski ES, Gaffey TA et al: Primary carcinoma of the fallopian tube. Am J Obstet Gynecol 154:1319, 1986 |
|
Peters WA, Anderson WA, Hopkins MP et al: Prognostic features of carcinoma of the fallopian tube. Obstet Gynecol 71:757, 1988 |
|
Kosary C, Trimble EL: Treatment and survival for women with fallopian tube carcinoma: a population-based study. Gynecol Oncol 86:190, 2002 |
|
Vinall PS, Buxton N: Primary carcinoma of the fallopian tube associated with tuberculous salpingitis: A case report. Br J Obstet Gynaecol 86:984, 1979 |
|
Sedlis A: Primary carcinoma of the fallopian tube. Obstet Gynecol Surv 16:209, 1961 |
|
Yoonessi M, Leberer JP, Crickard K: Primary fallopian tube carcinoma: Treatment and spread pattern. J Surg Oncol 38:97, 1988 |
|
Benedet JL, White GW, Fairey RN et al: Adenocarcinoma of the fallopian tube: Experience with 41 patients. Obstet Gynecol 50:654, 1977 |
|
Paley PJ, Swisher EM, Garcia RL et al: Occult cancer of the fallopian tube in BRCA-1 germline mutation carriers at prophylactic oophorectomy: a case for recommending hysterectomy at surgical prophylaxis. Gynecol Oncol 80:176, 2001 |
|
Aziz S, Kuperstein G, Rosen B et al: A genetic epidemiological study of carcinoma of the fallopian tube. Gyn Onc 80:341, 2001 |
|
Ingram FH, Hisley JC: Primary carcinoma of the fallopian tube. South Med J 68:1153, 1975 |
|
Markman M, Zaino R, Busowski J et al: Carcinoma of the fallopian tube. In: Hoskins W, Perez C, Young R, (eds): Principles and Practice of Gynecologic Oncology. p 783, Philadelphia, JB Lippincott, 1992 |
|
Kinzel GE: Primary carcinoma of the fallopian tube. Am J Obstet Gynecol 125:816, 1976 |
|
McMurray EH, Jacobs AJ, Perez CA et al: Carcinoma of the fallopian tube: Management and sites of failure. Cancer 58:2070, 1986 |
|
Pauerstein CJ, Woodruff JD: Cellular patterns in proliferative and anaplastic disease of the fallopian tube. Am J Obstet Gynecol 96:486, 1966 |
|
Rose PG, Piver MS, Tsukada Y: Fallopian tube cancer. Cancer 66:2661, 1990 |
|
Schiller HM, Siverberg SC: Staging and prognosis in primary carcinoma of the fallopian tube. Cancer 28:389, 1971 |
|
Hu CY, Taymor ML, Hertig AT: Primary carcinoma of the fallopian tube. Am J Obstet Gynecol 59:58, 1950 |
|
Puls LE, Davey DD, DePriest PD et al: Immunohistochemical staining for Ca-125 in Fallopian tube carcinomas. Gynecol Oncol 48:360, 1993 |
|
Uehira K, Hashimoto J, Tsuneyoshi M et al: Transitional cell carcinoma pattern in primary carcinoma of the fallopian tube. Cancer 72:2447, 1993 |
|
Jackson-York GL, Ramzy I: Synchronous papillary mucinous adenocarcinoma of the endocervix and fallopian tubes. Int J Gynecol Pathol 11:63, 1992 |
|
Malinak LJ, Miller G, Armstrong J: Primary squamous cell carcinoma of the fallopian tube. Am J Obstet Gynecol 95:1067, 1966 |
|
Boet R, Lifshitz S: Primary clear cell adenocarcinoma of the fallopian tube: Light microscopic and ultrastructural findings. Int J Gynecol Pathol 1:292, 1982 |
|
Silva E, Robey S, Smith T et al: Ovarian carcinomas with transitional cell carcinoma pattern. Am J Clin Pathol 93:457, 1990 |
|
Dekel A, Van Iddekinge B, Isaacson C et al: Primary choriocarcinoma of the fallopian tube: Report of a case with survival and postoperative delivery: Review of the literature. Obstet Gynecol Surv 41:142, 1986 |
|
Bakri YN, Amiri A, Mulla J: Gestational choriocarcinoma in a tubal ectopic pregnancy. Acta Obstet Gynecol Scand 71:67, 1992 |
|
Griggith L, Carcangiu ML: Sex cord tumor with annular tubules associated with endometriosis of the fallopian tube. Am J Clin Pathol 96:259, 1991 |
|
Hoda S, Huvos A: Struma salpingis associated with struma ovarii. Am J Surg Pathol 17:1187, 1993 |
|
Hanjani P, Petersen RO, Bonnell SA: Malignant mixed mullerian müllerian tumor of the fallopian tube. Gynecol Oncol 9:381, 1980 |
|
Carlson JA, Ackerman BL, Wheeler JE: Malignant mixed mullerian müllerian tumor of the fallopian tube. Cancer 71:187, 1993 |
|
Weber A, Hewett W, Gajewski W et al: Malignant mixed mullerian müllerian tumors of the fallopian tube. Gynecol Oncol 50:239, 1993 |
|
Abrams J, Kazal HL, Hobbs RE: Primary sarcoma of the fallopian tube. Am J Obstet Gynecol 75:180, 1958 |
|
Scheffey LC, Lang WR, Nugent FB: Clinical and pathologic aspects of the uterine tube. Am J Obstet Gynecol 52:904, 1941 |
|
Jacoby AF, Fuller AF, Thor AD et al: Primary leiomyosarcoma of the fallopian tube. Gynecol Oncol 51:404, 1993 |
|
Young RH, Scully SE: Ovarian tumors of probable wolffian origin: A report of 11 cases. Am J Surg Pathol 7:125, 1983 |
|
Woodruff JD: Tumors of the fallopian tube and tumors and cysts of the pelvic ligaments and paraadnexal structures. In: Sciarra JJ (ed): Gynecology and Obstetrics. p 1, Vol 4, Chap 46:Philadelphia, JB Lippincott, 1994 |
|
Ross WM: Primary carcinoma of the ovary: A review of 150 cases, with an appraisal of the fallopian tube as a pathway of spread. Can Med Assoc J 94:1035, 1966 |
|
Kanbour AI, Stock RJ: Squamous cell carcinoma in situ of the endometrium and fallopian tube as superficial extension of invasive cervical carcinoma. Cancer 42:570, 1978 |
|
Andriole GL, Garnick MB, Richie JP: Unusual behavior of low-grade, low stage transitional cell carcinoma of the bladder. Urology 25:524, 1985 |
|
Case TC: Cancer of the breast with metastasis to the fallopian tube. J Am Geriatr Soc 16:832, 1968 |
|
Sedlis A: Carcinoma of the fallopian tube. Surg Clin North Amer 58:121, 1978 |
|
Plentl AA, Friedman EA: Lymphatic system of the female genitalia. Major Problems in Obstetrics and Gynecology. Vol 2:Philadelphia, WB Saunders, 1971 |
|
Tamini HK, Figge DC: Adenocarcinoma of the uterine tube: Potential for lymph node metastases. Am J Obstet Gynecol 141:132, 1981 |
|
Schray MF, Podratz KC, Malkasian GD: Fallopian tube cancer: The role of radiation therapy. Radiother Oncol 10:267, 1987 |
|
Maxson WZ, Stehman FB, Ulbright TM et al: Primary carcinoma of the fallopian tube: Evidence for activity of cisplatin combination therapy. Gynecol Oncol 26:305, 1987 |
|
Klein M, Rosen A, Lahousen M et al: Lymphogenous metastasis in the primary carcinoma of the fallopian tube. Gynecol Oncol 55:336, 1994 |
|
Barakat BR, Rubin SC, Saigo PE et al: Cisplatin-based combination chemotherapy in carcinoma of the fallopian tube. Gynecol Oncol 42:156, 1991 |
|
Asmussen M, Kaern J, Kjoerstad K et al: Primary adenocarcinoma localized to the fallopian tubes: Report of 33 cases. Gynecol Oncol 30:183, 1988 |
|
Semrad N, Watring W, Fu YS et al: Fallopian tube adenocarcinoma: Common extraperitoneal recurrence. Gynecol Oncol 24:230, 1986 |
|
Latzko W: Linkseitiges Tubenkarzinom rechtseitige karzinomatose tubo-ovarial cyste. Zentralbl Gynakol 40:599, 1916 |
|
Yoonessi M: Carcinoma of the fallopian tube. Obstet Gynecol Surv 34:257, 1979 |
|
Yeung HH, Bannatye P, Russell P: Adenocarcinoma of the fallopian tubes: A clinicopathological study of eight cases. Pathology 15:279, 1983 |
|
Muntz HG, Goff BA, Thor AD et al: Posthysterectomy carcinoma of the fallopian tube mimicking a vesicovaginal fistula. Obstet Gynecol 79:853, 1992 |
|
Hirai Y, Kaku S, Teshima H et al: Clinical study of primary carcinoma of the fallopian tube: Experience with 15 cases. Gynecol Oncol 34:20, 1989 |
|
Muntz HG, Taraza HM, Granai CO et al: Primary adenocarcinoma of the fallopian tube. Eur J Gynecol Oncol 10:239, 1989 |
|
Henderson S, Harper R, Salazar O et al: Primary carcinoma of the fallopian tube: Difficulties of diagnosis and treatment. Gynecol Oncol 5:168, 1977 |
|
Curtin J, Radden B: Mandibular metastasis from a primary adenocarcinoma of the fallopian tube. J Oral Maxillofac Surg 43:636, 1985 |
|
Galle P, Jogson V, Homesley H: Umbilical metastasis from gynecologic malignancies: A primary carcinoma of the fallopian tube. Obstet Gynecol 57:531, 1981 |
|
Campagnutta E, Scarabelli C, Juzzolino C: Fallopian tube carcinoma: Report of an unusual clinical case. Eur J Gynaecol Oncol 2:134, 1981 |
|
Carapeto R, Nogales FF, Matilla A: Ectopic pregnancy coexisting with a primary carcinoma of the fallopian tube: A case report. Int J Gynaecol Obstet 16:263, 1978 |
|
Ajjimakorn S, Bhamarapravati Y, Israngura N: Ultrasound appearance of fallopian tube carcinoma. J Clin Ultrasound 16:516, 1988 |
|
Ajjimakorn S, Bhamarapravati Y: Transvaginal ultrasound and the diagnosis of fallopian tubal carcinoma. J Clin Ultrasound 19:116, 1991 |
|
Granberg S, Jansson I: Early detection of primary carcinoma of the fallopian tube by endovaginal ultrasound. Acta Obstet Gynecol Scand 69:667, 1990 |
|
Yuen JHF, Wong GCY, Lam CHL: Preoperative sonographic diagnosis of primary fallopian tube carcinoma. J Ultrasound Med 21:1171, 2002 |
|
Karlan BY, Hoh C, Tse N et al: Whole-body positron emission tomography with (fluorine-18)-2-deoxyglucose can detect metastatic carcinoma of the fallopian tube. Gynecol Oncol 49:383, 1993 |
|
Hirai Y, Chen J-T, Hamanda T et al: Clinical and cytologic aspects of primary fallopian tube carcinoma: A report of ten cases. Acta Cytol 31:834, 1987 |
|
Takashina T, Ito E, Kudo R: Cytologic diagnosis of primary tubal cancer. Acta Cytol 29:367, 1985 |
|
Erez S, Kaplan AL, Wall JA: Clinical staging of carcinoma of the uterine tube. Obstet Gynecol 30:547, 1967 |
|
Dodson JB, Ford JH, Averette HE: Clinical aspects of fallopian tube carcinoma. Obstet Gynecol 36:935, 1970 |
|
Pettersson F: Staging rules for gestational trophoblastic tumors and fallopian tube cancer. Acta Obstet Gynecol Scand 71:224, 1992 |
|
Rosen AC, Reiner A, Klein M et al: Prognostic factors in primary fallopian tube carcinoma. Gynecol Oncol 53:307, 1994 |
|
Rosen AC, Graf AH, Klein M et al: DNA ploidy in primary fallopian tube carcinoma using imagery cytometry. Int J Cancer 58:362, 1994 |
|
Morris M, Gershenson GM, Burke TW et al: Treatment of fallopian tube carcinoma with cisplatin, doxorubicin and cyclophosphamide. Obstet Gynecol 76:1020, 1990 |
|
Querleu D, LeBlanc E: Laparoscopic infrarenal paraaortic lymph node dissection for restaging of carcinoma of the ovary or fallopian tube. Cancer 73:1467, 1994 |
|
Gurney H, Murphy D, Crowther D: The management of primary fallopian tube carcinoma. Br J Obstet Gynaecol 97:822, 1990 |
|
Harrison C, Averette H, Jarrell M et al: Carcinoma of the fallopian tube: Clinical management. Gynecol Oncol 32:357, 1989 |
|
Roberts J, Lifshitz S: Primary adenocarcinoma of the fallopian tube. Gynecol Oncol 13:301, 1982 |
|
Jacobs A, McMurray E, Parham J et al: Treatment of carcinoma of the fallopian tube using cisplatin, doxorubicin and cyclophosphamide. Am J Clin Oncol 9:436, 1986 |
|
Deppe G, Bruckner HW, Cohen CJ: Combination chemotherapy for advanced carcinoma of the fallopian tube. Obstet Gynecol 56:530, 1980 |
|
Peters WA, Anderson WA, Hopkins MP: Results of chemotherapy in advanced carcinoma of the fallopian tube. Cancer 63:836, 1989 |
|
Brown M, Kohorn E, Kapp D et al: Fallopian tube carcinoma. Int J Radiat Oncol Biol Phys 11:583, 1985 |
|
Barakat R, Rubin S, Saigo P et al: Second-look laparotomy in carcinoma of the fallopian tube. Obstet Gynecol 82:748, 1993 |
|
Pectasides D, Barbounis V, Sintila A et al: Treatment of primary fallopian tube carcinoma with cisplatin-containing chemotherapy. Am J Clin Oncol 17:68, 1994 |
|
Phelps HM, Chapman KE: Role of radiation therapy in treatment of primary carcinoma of the uterine tube. Obstet Gynecol 43:669, 1974 |
|
Markman M, Glass T, Smith HO et al: Phase II trial of single agent carboplatin followed by dose-intense paclitaxel, followed by maintenance paclitaxel therapy in stage IV ovarian, fallopian tube, and peritoneal cancers: A Southwest Oncology Group trial. Gyn Onc 88:3:282, 2003 |
|
Markman M, Kennedy A, Webster K et al: Combination chemotherapy with carboplatin and docetaxel in the treatment of cancers of the ovary and fallopian tube and primary carcinoma of the peritoneum. J Clin Oncol. 19:7:1901, 2001 |
|
Rose PG, Fusco N, Smrekar M et al: Successful administration of carboplatin in patients with clinically documented carboplatin hypersensitivity. Gynecol Oncol 89:429, 2003 |
|
Dizon DS, Sabbatini PJ, Aghajanian C et al: Analysis of patients with epithelial ovarian cancer or fallopian tube carcinoma retreated with cisplatin after the development of a carboplatin allergy. Gynecol Oncol 84:378, 2002 |
|
Gemignani ML, Hensley ML, Cohen R et al: Paclitaxel-based chemotherapy in carcinoma of the fallopian tube. Gynecol Oncol 80:16, 2001 |
|
McGuire W, Hoskins W, Brady M et al: Taxol and cisplatin (TP) improves outcome in advanced ovarian cancer (AOC) (abstr 771). Proc Am Soc Clin Oncol 14:275, 1995 |
|
Christian M, Trimble E: Salvage chemotherapy for epithelial ovarian carcinoma. Gynecol Oncol 55:suppl S143, 1994 |
|
Gershenson D, Burke T, Morris M et al: A phase I trial of intravenous melphalan, Taxol, and cisplatin + G-CSF in patients with suboptimal advanced ovarian cancer, fallopian tube cancer or serous carcinoma of the peritoneum (abstr 787). Proc Am Soc Clin Oncol 14:279, 1995 |
|
Chalmers JA, Marshall AT: Carcinoma of the fallopian tube. Br J Obstet Gynaecol 83:580, 1976 |